
transcranial Electrical Stimulation (tES) device
Neurophet innk is a non-invasive electrical stimulation device designed to aid in the functional
recovery and rehabilitation of patients with brain diseases.

tES device aiding in functional recovery of brain through electrical stimulation
Neurophet innk helps patients recover from depression and stroke through
electrical stimulation.
Enhances attention and working memory of patients on antidepressant medication for depression, and also
improves post-stroke finger movement impairment in conjunction with rehabilitation.

Features
Non-invasive
electrical stimulation
tES treatment has fewer side effects and high patient compliance.
Micro electrical
stimulation using electrodes
Provides precise treatment using various sizes of electrodes.
Portable
device
Small sized and lightweight device to conveniently carry with.
Easy
to use
Able to manage stimulation setting and log through innk App.
innk App helps set up a personalized treatment plan for patients.
Manages 5 devices simultaneously
Saves maximum 5,000 stimulation logs
View patient compliance (Provides CSV report)
Customizes the treatment settings for the patient’s therapeutic environment. (max daily stimulation sessions, inter-stimulation rest intervals, etc.)
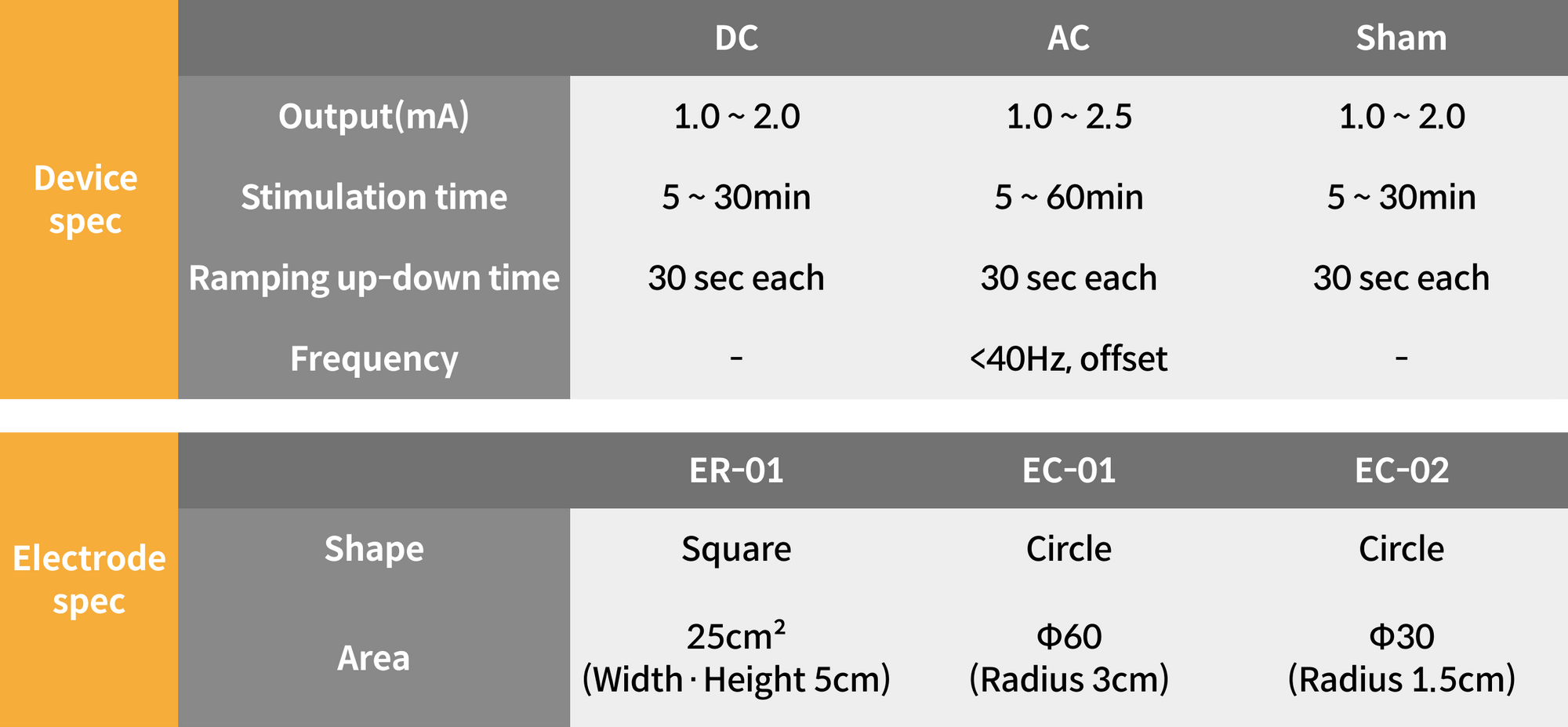
Stimulation parameters (DC/AC/Sham) adapted for the treatment plan

Clinical Evidence
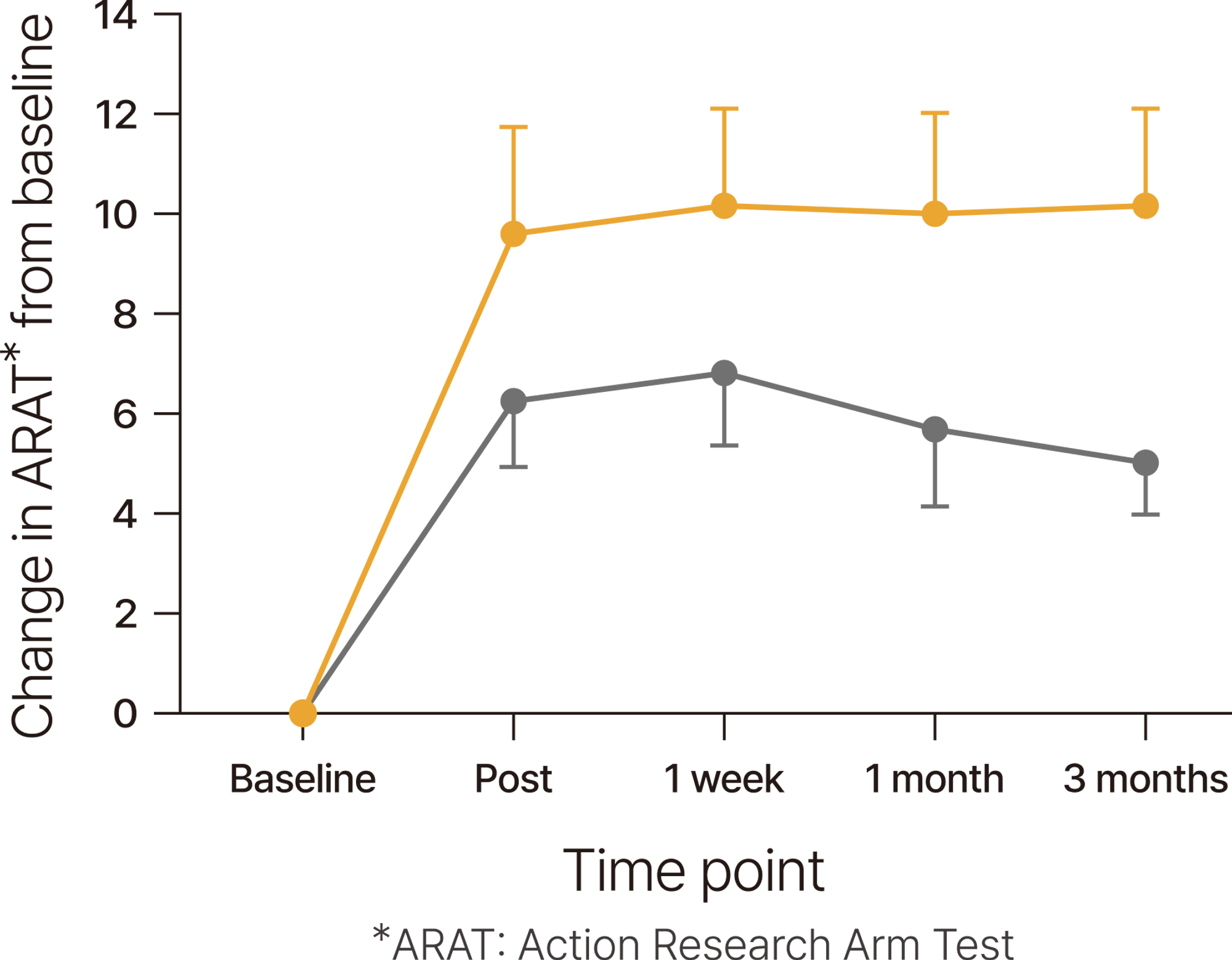
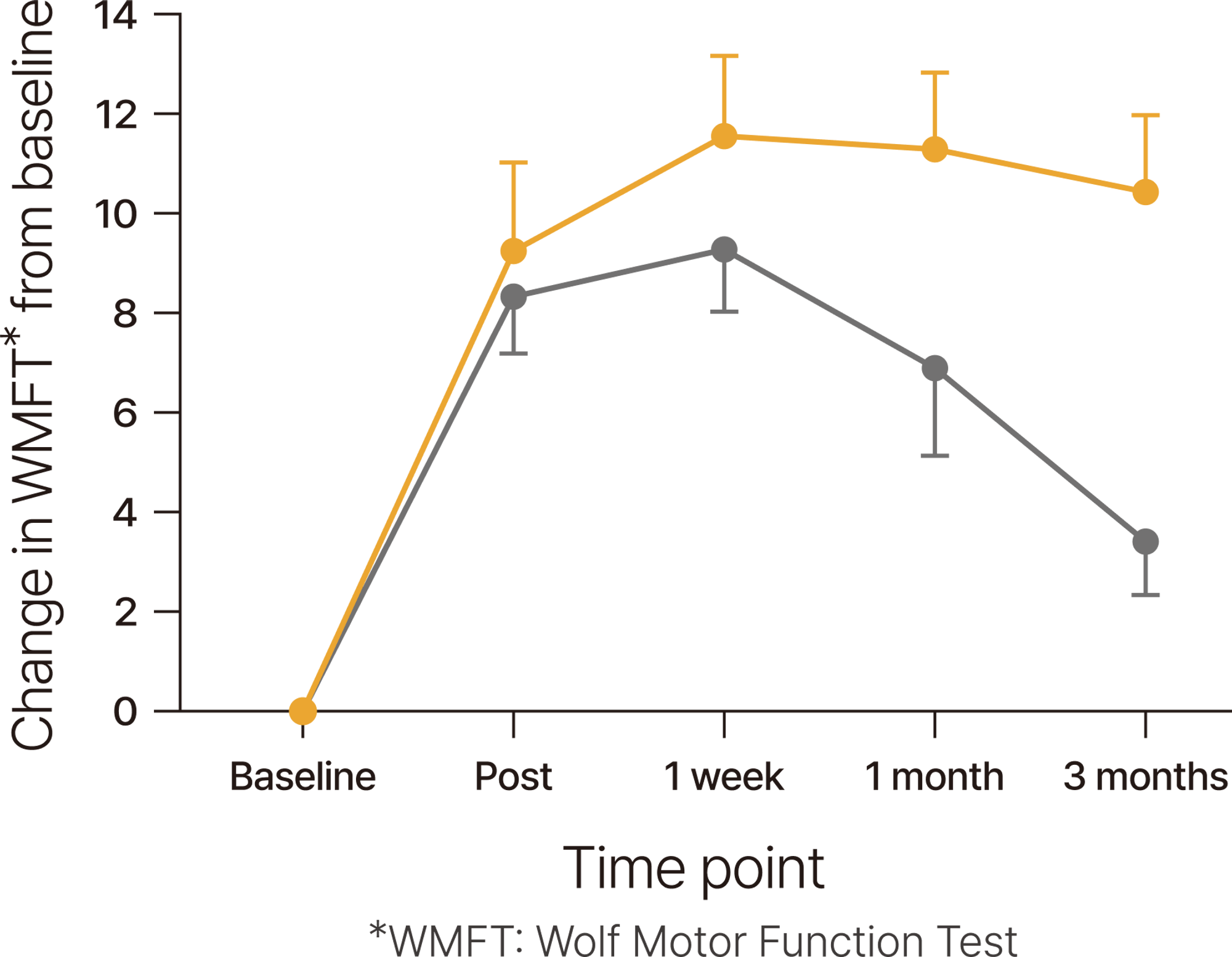
When combined with rehabilitation, tDCS* helps maintain improved motor function for three months.
Patients in the acute to chronic stages of stroke experienced upper limb function improvement with 9 sessions of tDCS (1mA, 20 minutes each), in parallel with exercise, and this post-treatment effect was sustained for up to three months.
*tDCS: transcranial Direct Current Stimulation
*Ref. Allman 2016, Science Translational Medicine / DOI: 10.1126/scitranslmed.aad5651
a randomized, controlled trial in 24 patients(anodal tDCS (n=11), sham treatment (n=13) paired with daily motor training for 9 days) at least 6 months after a first unilateral stroke not directly involving the primary motor cortex
Technical Specifications

Product information
Product
Electroencephalograph
Model
innk01-DW
Product Approval No.
21-621 (MFDS, Class lll)

Experience electrical stimulation(tES) device, Neurophet innk
* Only medical personnel can request a demo for Neurophet innk.